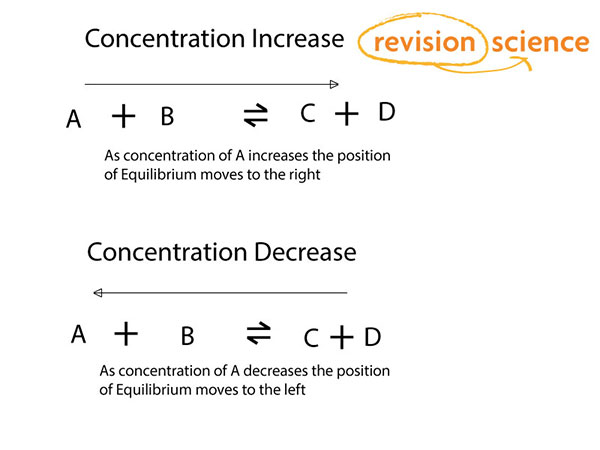
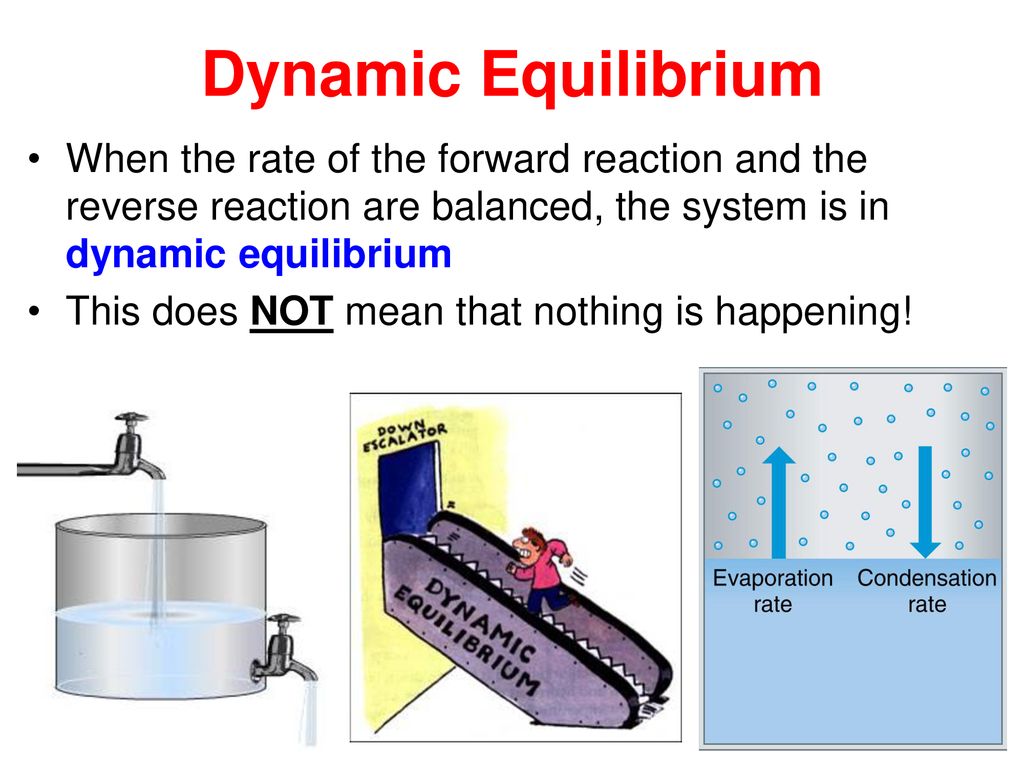
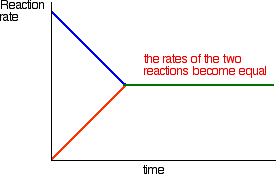
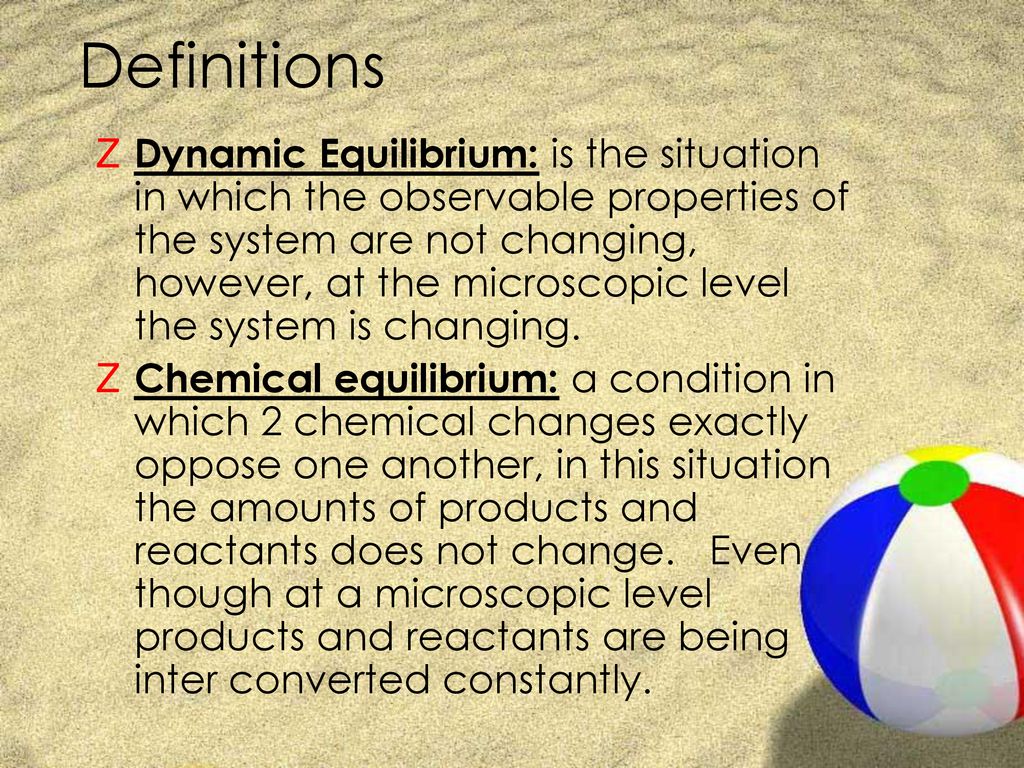
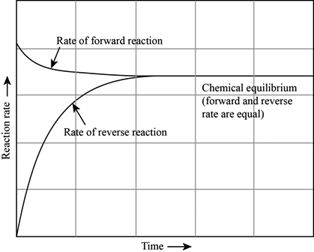
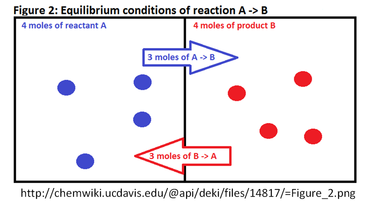
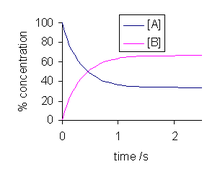
Chapter 12: Chemical Equilibrium. The Dynamic Nature of Equilibrium A. What is equilibrium? 1. Definition a state of balance; no net change in a dynamic. - ppt download

Chemical Equilibria: Reversible Reactions, Dynamic Equilibrium (1.7.1) | CIE AS Chemistry Revision Notes 2019 | Save My Exams

Difference Between Chemical Equilibrium and Dynamic Equilibrium | Compare the Difference Between Similar Terms
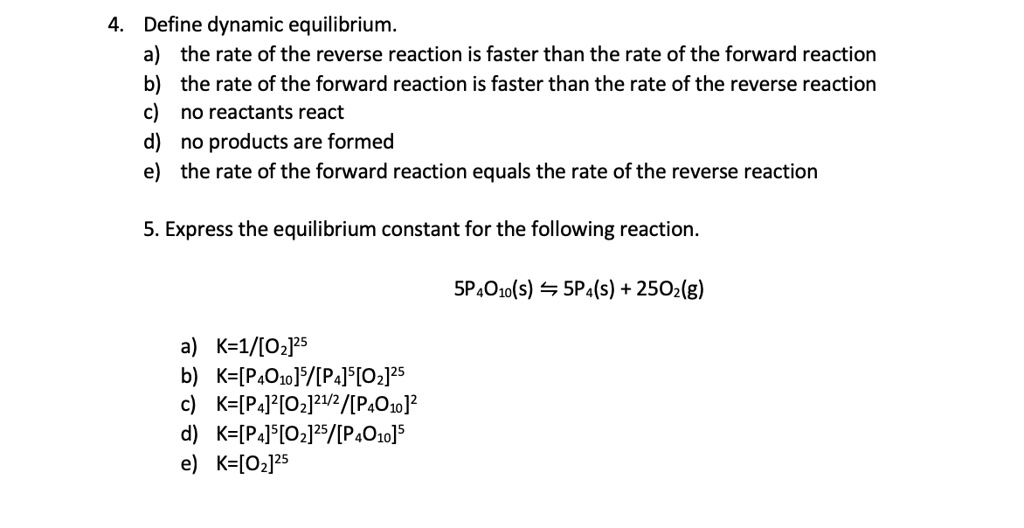
SOLVED: Define dynamic equilibrium: a) the rate of the reverse reaction is faster than the rate of the forward reaction the rate of the forward reaction is faster than the rate of

SOLVED:Explain dynamic equilibrium with respect to solution formation. What is a saturated solution? An unsaturated solution? A supersaturated solution?