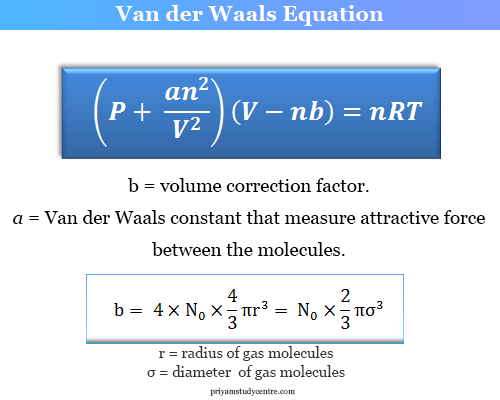
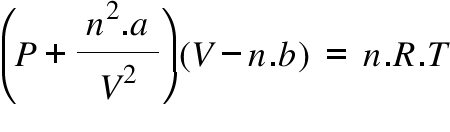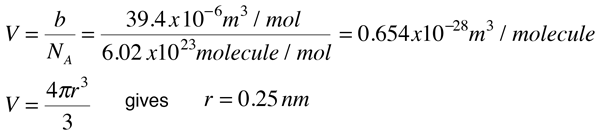Calculate the radius of He atoms if its van der Waal's constant 'b' is 24mL `"mol"^(-1)`. (Note: mL= - YouTube
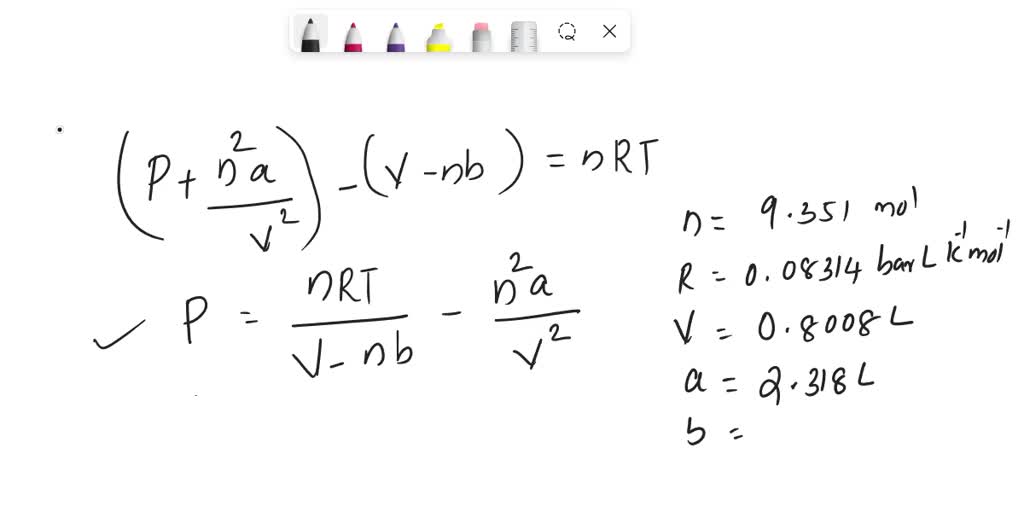
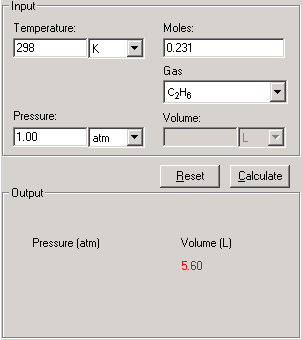
SOLVED: Calculate pressure using the ideal gas law and the van der Waals equation. A 1.67-mol sample of krypton gas is maintained in a 0.733-L container at 297 K. Calculate the pressure

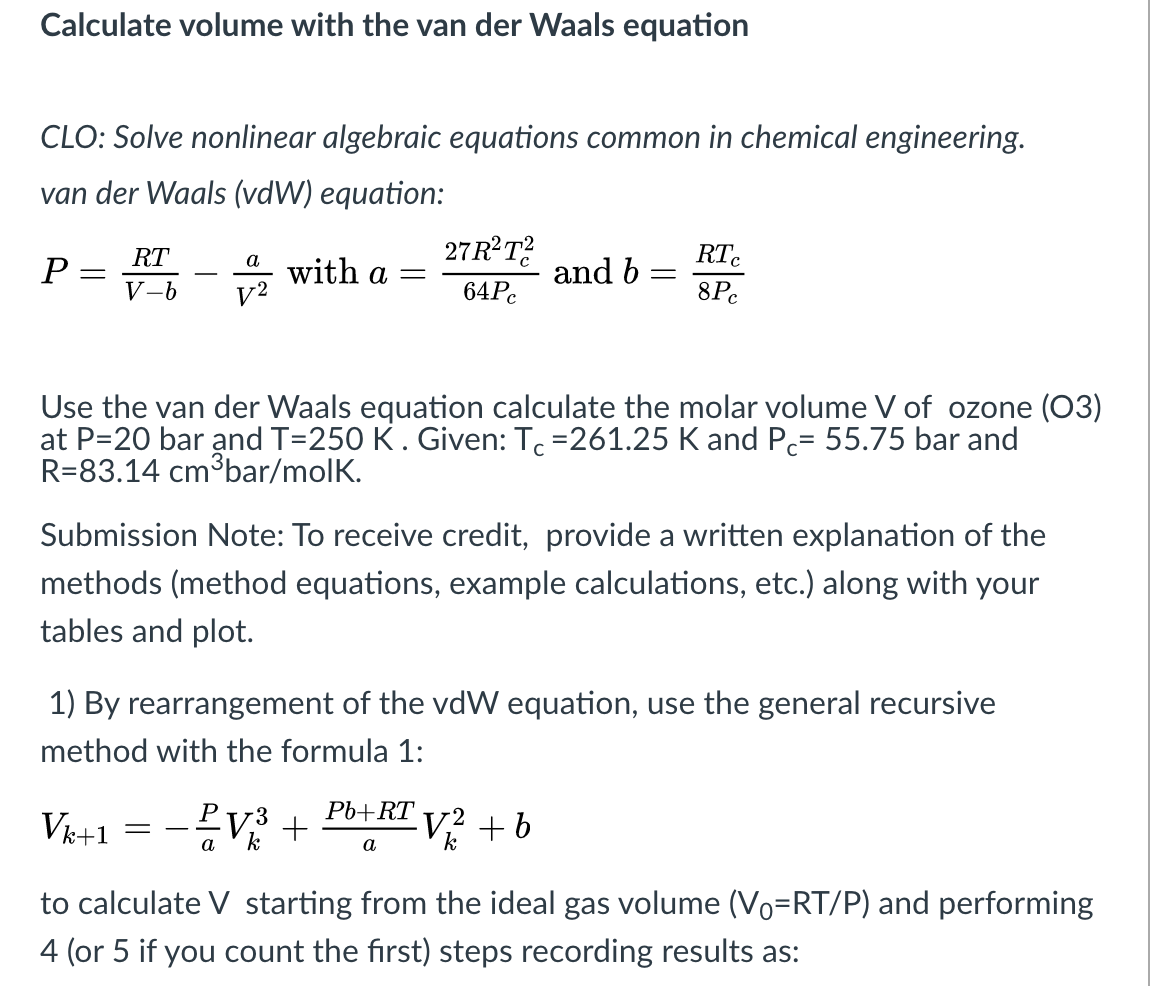
Calculate the pressure exerted by 22 g of carbon dioxide in 0.5 dm^3 at 300 K using:(a) Ideal gas law and(b) Van der Waals equation [Given: a = 360 kPa dm^6 mol^-2

a) Calculate the pressure exerted by 5 moles of CO2 in the one - liter vessel at 47^∘C using van der Waals' equation. Also, report the pressure of the gas if it

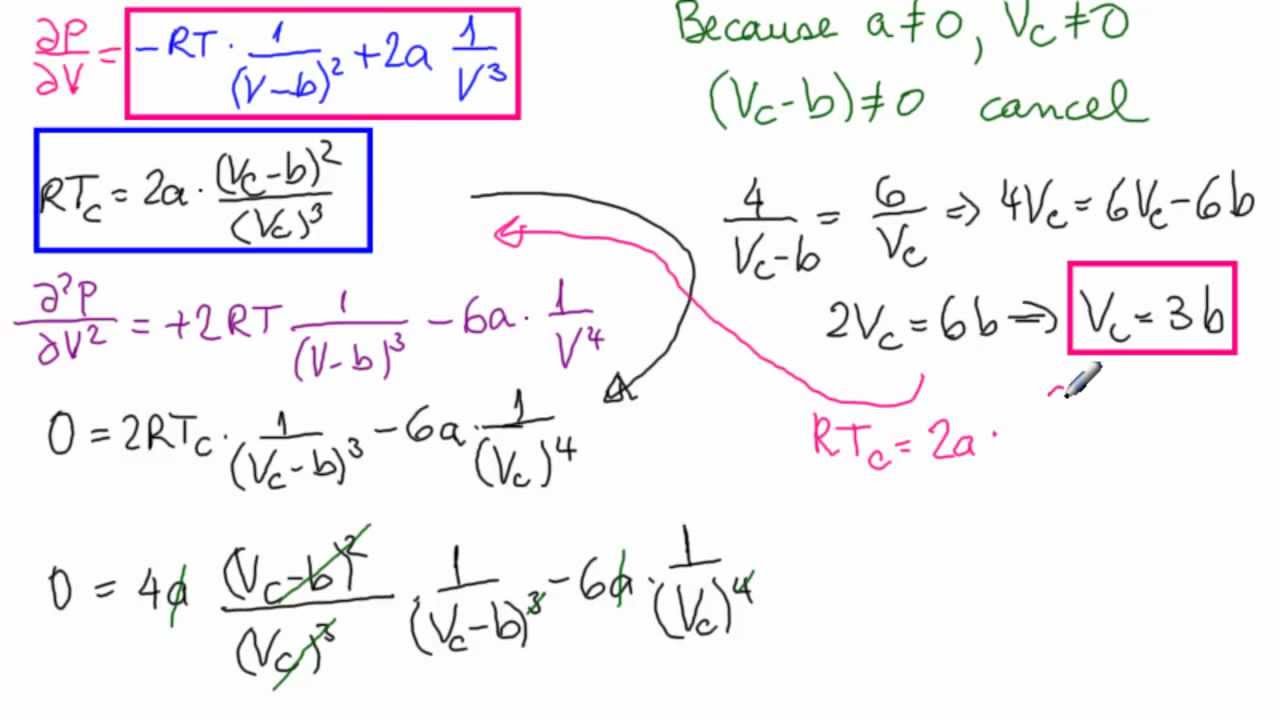
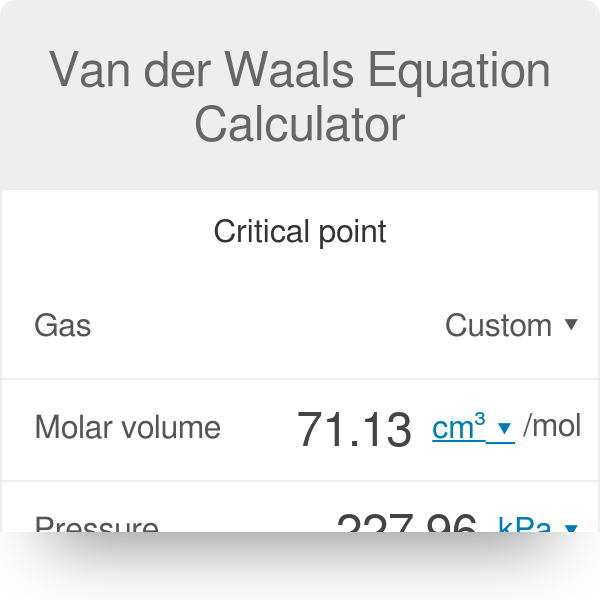
Calculate the Van der Waals constants for carbon dioxide if its critical temperature Tcr = 304 K and critical pressure pcr = 73 atm .