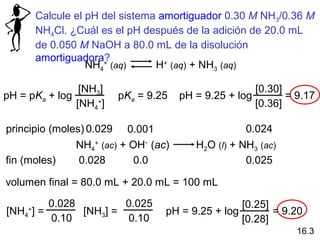
PPT – Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL of 0.050 M NaOH to 80.0 mL of

PPT – Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL of 0.050 M NaOH to 80.0 mL of

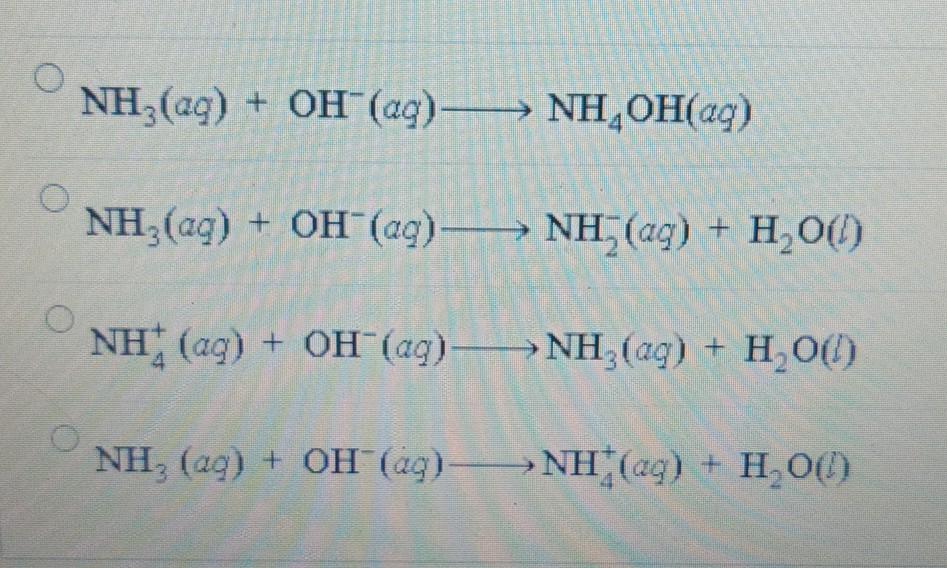
A buffer solution made with NH3 and NH4Cl has a pH of 10.0 which procedure could be used to lower the pH ? 1.Adding HCl , 2.Adding NH3 , 3. Adding NH4Cl
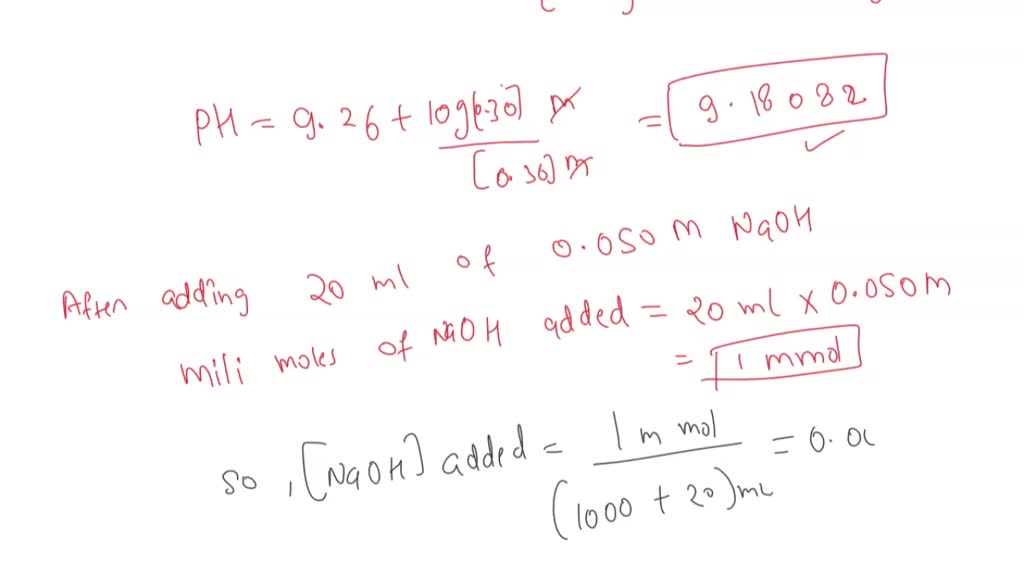
SOLVED: 1. Calculate the pH of the 0.30 M NH3/ 0.36M NH4Cl buffer system. What is the pH after addition of 20.0 mL of 0.050 M NaOh to 1L of solution. a.
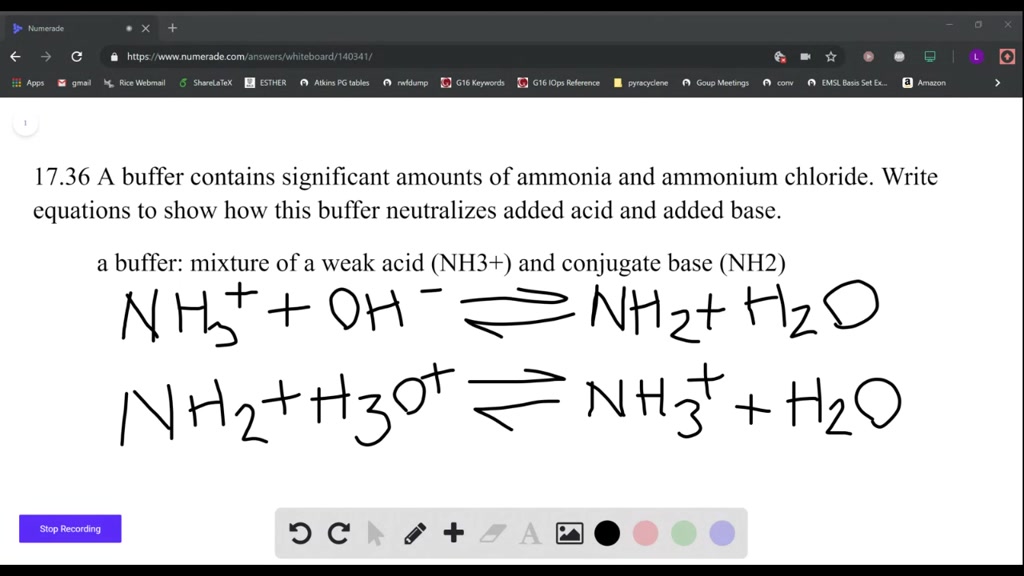
SOLVED: A buffer contains significant amounts of ammonia, nh3 , and ammonium chloride, nh4cl . part a write an equation showing how this buffer neutralizes added acid, hi.

a buffer solution contain NH3 and NH4CL total concentration of buffering agent is 0 6 molar if the pOH of - Chemistry - Equilibrium - 14195039 | Meritnation.com

Calculate the amount of NH3 and NH4Cl required to prepare a buffer solution of pH 9.0 when total concentration of buffering reagents is 0.6 mol L^- . pKb for NH3 = 4.7, log 2 = 0.30 .
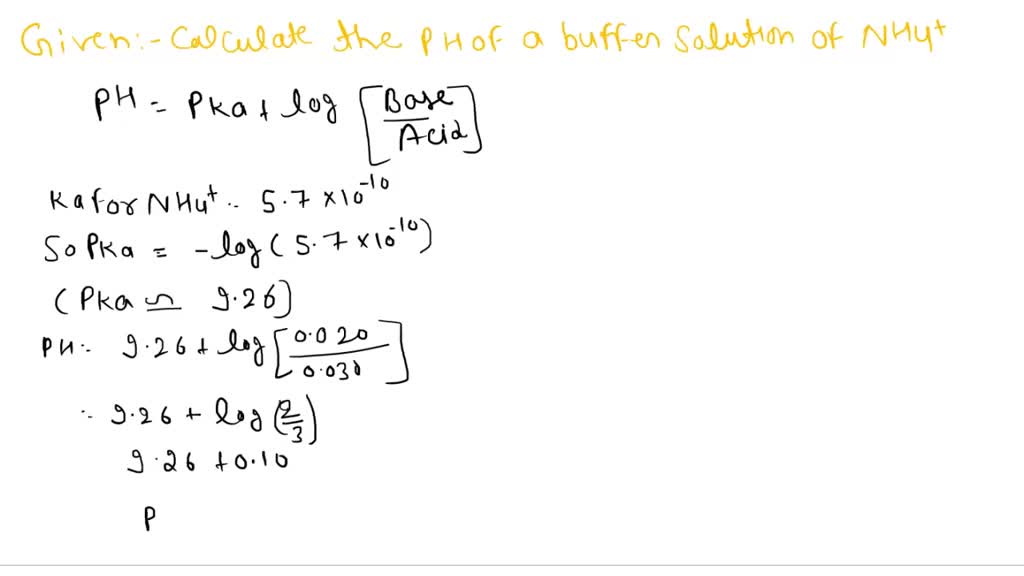
SOLVED: Calculate the pH of a buffer that is 0.020 M in NH3 and 0.030 M in NH4Cl. What is the pH after adding 1.00 mL of 0.01 M NaOH to 0.10
A buffer solution containing NH3 and NH4Cl has a pH value of 9. pKb for NH3 is 4.7. If in the buffer solution total concentration of buffering reagents is 0.6 mol L^(-1),